Reaction: CBL is tyrosine phosphorylated
- in pathway: Regulation of signaling by CBL
Cbl is tyrosine phosphorylated following stimulation with IL-3 (Anderson et al. 1997) and GM-CSF (Odai et al. 1995). Cbl may be phosphorylated prior to this IL-3 stimulated tyrosyl phosphorylation (Park et al. 1998). The kinase responsible for Cbl phosphorylation may be dependent on cell type; Fyn is demonstrated to have the ability to phosphorylate Cbl (Hunter et al. 1999), other candidates include Hck, Lyn (Hunter et al. 1999) and Syk (Park et al. 1998).
Tyrosines 700, 731 and 774 are the major sites of Cbl phosphorylation by non-receptor protein tyrosine kinases, with none showing any particular specificity for sites (Tsygankov et al. 2001). Fyn was observed to be constitutively associated with Cbl in lysates of several different cell types including the interleukin-3-dependent murine myeloid cell line 32Dcl3, and the prolactin-dependent rat thymoma cell line Nb2. Cbl phosphorylation at Y731 is postulated to provide an additional interaction between Cbl and the SH2 domain of p85-PI3K (Hunter et al. 1999). Cbl-p85 association increases in activated cells (Panchamoorthy et al. 1996). Expression of a Cbl Y731F mutant which abolishes binding of Cbl to p85 markedly increased levels of p85-PI3K (Dufour et al. 2008). Cbl-p85 binding negatively regulates PI3K activity (Fang et al. 2001); Cbl phosphorylation increased PI3K ubiquitination and proteasome degradation (Dufour et al. 2008). Cbl association with members of the Crk family is mediated by phosphorylation of Y700 and Y774 (Andoniou et al. 1996), binding with Vav is mediated by Y770 (Marengere et al 1997).
Tyrosines 700, 731 and 774 are the major sites of Cbl phosphorylation by non-receptor protein tyrosine kinases, with none showing any particular specificity for sites (Tsygankov et al. 2001). Fyn was observed to be constitutively associated with Cbl in lysates of several different cell types including the interleukin-3-dependent murine myeloid cell line 32Dcl3, and the prolactin-dependent rat thymoma cell line Nb2. Cbl phosphorylation at Y731 is postulated to provide an additional interaction between Cbl and the SH2 domain of p85-PI3K (Hunter et al. 1999). Cbl-p85 association increases in activated cells (Panchamoorthy et al. 1996). Expression of a Cbl Y731F mutant which abolishes binding of Cbl to p85 markedly increased levels of p85-PI3K (Dufour et al. 2008). Cbl-p85 binding negatively regulates PI3K activity (Fang et al. 2001); Cbl phosphorylation increased PI3K ubiquitination and proteasome degradation (Dufour et al. 2008). Cbl association with members of the Crk family is mediated by phosphorylation of Y700 and Y774 (Andoniou et al. 1996), binding with Vav is mediated by Y770 (Marengere et al 1997).
Reaction - small molecule participants:
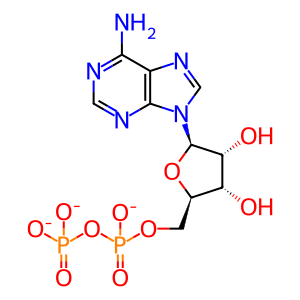
ADP [cytosol]
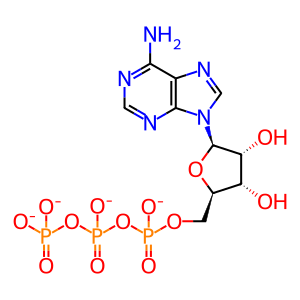
ATP [cytosol]
Reactome.org reaction link: R-HSA-912629
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-912629