Reaction: ALDH2 transforms GTN to NO
- in pathway: Smooth Muscle Contraction
In the mid-19th century, nitroglycerin (nitroglycerine, glyceryl trinitrate, GTN) was synthesised by the Italian chemist Ascanio Sobrero (1812–1888) and was discovered to be a powerful vasodilator (Marsh & Marsh 2000), as well as an active ingredient in the manufacture of explosives, mostly dynamite. It was first used to relieve angina in 1867 and was noted to have pharmacological resistance on repeated doses (Daiber & Münzel 2015). Today, GTN is still the treatment of choice for relieving angina. Other organic esters and inorganic nitrates are also used, but the rapid action of GTN and its established efficacy make it the mainstay of angina pectoris relief.
GTN relaxes blood vessels primarily via activation of the soluble guanylyl cyclase (sGC)/cGMP/cGMP-dependent protein kinase (cGK-I) pathway (see reviews Münzel et al. 2003, Daiber & Münzel 2015). The activation of sGC by nitrate-derived nitric oxide (NO) and/or S-nitrosothiol was identified as the principal mechanism of action of organic nitrates such as GTN (Schulz et al. 2002, Opelt et al. 2016). Nitrate generated within the mitochondria is metabolised further by reduction to NO and/or by conversion to S-nitrosothiol (Chen & Stamler 2006, Chen et al. 2002). However, the exact reaction mechanism of this mitochondrial NO production, as well as the NO form which is conveyed from mitochondria to cytosolic sGC, remain unresolved (Mayer & Beretta 2008, Chen & Stamler 2006). Nitrate drug biotransformation pathways to NO and their effects as NO donors across tissues remain ill defined. GTN-induced vasodilation might be mediated by an NO-related species, for example, iron–nitrosyl or S-nitroso species. A significant increase in iron–nitrosyl or S-nitroso species can be seen minutes after oral intake of GTN in human volunteers and animals (Wenzel et al. 2009, Janero et al. 2004).
Mitochondrial aldehyde dehydrogenase 2 (ALDH2) catalyses the bioactivation of GTN by the formation of a reactive NO or NO-related intermediate that activates sGC. Conversion of GTN into GDN (1,2-glycerol dinitrate) and nitrite by mitochondrial ALDH2 may be an essential pathway of GTN bioactivation in blood vessels (Kollau et al. 2005). NO binds to a heme group of sGC. Activation of sGC leads to increased bioavailability of cyclic guanosine-3′,-5′-monophosphate (cGMP) and activation of cGMP-dependent protein kinases, such as the cGMP-dependent protein kinase I (cGK-I). cGK-I inhibits the inositol-1,4,5-trisphosphate (IP3)-dependent calcium release from the ER, inhibiting myosin light chain kinase (MLCK) and thereby vasoconstriction. cGKI also phosphorylates Rho and interferes with Rho kinase inhibition of myosin light chain phosphatase (MLCP). MLCP dephosphorylates MLC and enhances SMC relaxation. It is well known that GTN can inhibit mitochondrial ALDH2 activity (Mukerjee & Pietruszko 1994). Chen et al. (Chen et al. 2002) were the first to provide evidence that prolonged treatment with GTN results in GTN tolerance and simultaneous inhibition of mitochondrial ALDH2 in vascular preparations.
For simplification, the probable NO production from GTN is described as a single event, collating the probable denitrification and reduction steps involved.
GTN relaxes blood vessels primarily via activation of the soluble guanylyl cyclase (sGC)/cGMP/cGMP-dependent protein kinase (cGK-I) pathway (see reviews Münzel et al. 2003, Daiber & Münzel 2015). The activation of sGC by nitrate-derived nitric oxide (NO) and/or S-nitrosothiol was identified as the principal mechanism of action of organic nitrates such as GTN (Schulz et al. 2002, Opelt et al. 2016). Nitrate generated within the mitochondria is metabolised further by reduction to NO and/or by conversion to S-nitrosothiol (Chen & Stamler 2006, Chen et al. 2002). However, the exact reaction mechanism of this mitochondrial NO production, as well as the NO form which is conveyed from mitochondria to cytosolic sGC, remain unresolved (Mayer & Beretta 2008, Chen & Stamler 2006). Nitrate drug biotransformation pathways to NO and their effects as NO donors across tissues remain ill defined. GTN-induced vasodilation might be mediated by an NO-related species, for example, iron–nitrosyl or S-nitroso species. A significant increase in iron–nitrosyl or S-nitroso species can be seen minutes after oral intake of GTN in human volunteers and animals (Wenzel et al. 2009, Janero et al. 2004).
Mitochondrial aldehyde dehydrogenase 2 (ALDH2) catalyses the bioactivation of GTN by the formation of a reactive NO or NO-related intermediate that activates sGC. Conversion of GTN into GDN (1,2-glycerol dinitrate) and nitrite by mitochondrial ALDH2 may be an essential pathway of GTN bioactivation in blood vessels (Kollau et al. 2005). NO binds to a heme group of sGC. Activation of sGC leads to increased bioavailability of cyclic guanosine-3′,-5′-monophosphate (cGMP) and activation of cGMP-dependent protein kinases, such as the cGMP-dependent protein kinase I (cGK-I). cGK-I inhibits the inositol-1,4,5-trisphosphate (IP3)-dependent calcium release from the ER, inhibiting myosin light chain kinase (MLCK) and thereby vasoconstriction. cGKI also phosphorylates Rho and interferes with Rho kinase inhibition of myosin light chain phosphatase (MLCP). MLCP dephosphorylates MLC and enhances SMC relaxation. It is well known that GTN can inhibit mitochondrial ALDH2 activity (Mukerjee & Pietruszko 1994). Chen et al. (Chen et al. 2002) were the first to provide evidence that prolonged treatment with GTN results in GTN tolerance and simultaneous inhibition of mitochondrial ALDH2 in vascular preparations.
For simplification, the probable NO production from GTN is described as a single event, collating the probable denitrification and reduction steps involved.
Reaction - small molecule participants:
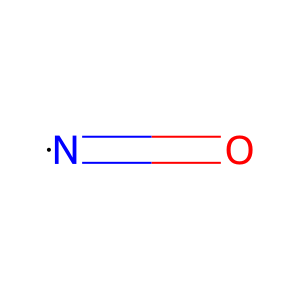
NO [mitochondrial matrix]
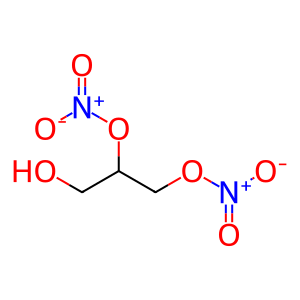
GDN [mitochondrial matrix]
H2O [mitochondrial matrix]
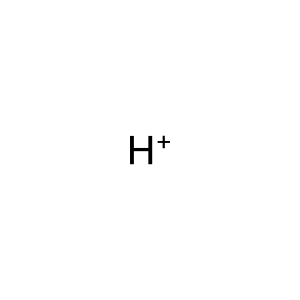
H+ [mitochondrial matrix]
Reactome.org reaction link: R-HSA-9620103
======
Reaction input - small molecules:
hydron
Reaction output - small molecules:
nitric oxide
1,2-dinitroglycerol
water
Reactome.org link: R-HSA-9620103