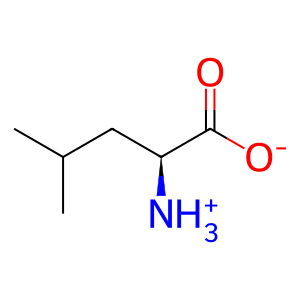
Reaction: SESN1,2 binds L-leucine and dissociates from GATOR2
- in pathway: Amino acids regulate mTORC1
SESN2 (Sestrin2) and SESN1 (Sestrin1) each interact with the GATOR2 complex in the absence of leucine and, upon binding leucine, dissociate from the GATOR2 complex (Chantranupong et al. 2014, Parmigiani et al. 2014, Kim et al. 2015, Wolfson et al. 2016, Saxton et al. 2016). (SESN3 constitutively binds GATOR2 in the presence and absence of leucine.) When bound to GATOR2, the Sestrins appear to prevent GATOR2 from inhibiting GATOR1, a GTPase activator which negatively regulates mTORC1 activation by increasing the rate of hydrolysis of RRAGA,B:GTP to RRAGA,B:GDP. SESN1,2 complexed with GATOR2 therefore allows GATOR1 to maintain mTORC1 in the inactive state (Chantranupong et al. 2014, Parmigiani et al. 2014, Kim et al. 2015). L-leucine binds SESN1,2 and causes SESN1,2 to dissociate from GATOR2, allowing GATOR2 to inhibit GATOR1 and thereby maintain RRAGA,B in the active (GTP-bound) state (Wolfson et al. 2016, Saxton et al. 2016). GATOR1 is recruited to the lysosomal membrane by the KICSTOR complex (Wolfson et al. 2017, Peng et al. 2017).
Sestrins also appear to interact with the Rag heterodimer (Peng et al. 2014) though the interaction may be indirect (Budanov 2015).
Sestrins also appear to interact with the Rag heterodimer (Peng et al. 2014) though the interaction may be indirect (Budanov 2015).
Reaction - small molecule participants:
L-Leu [cytosol]
Reactome.org reaction link: R-HSA-9639287
======
Reaction input - small molecules:
L-leucine zwitterion
Reaction output - small molecules:
Reactome.org link: R-HSA-9639287