Reaction: GGCX does not gamma-carboxylate 3D-F9(29-461) (pro-factor IX)
Being a vitamin K‐dependent (VKD) glycoprotein, factor IX (FIX) is synthesized first as a precursor protein which then undergoes extensive posttranslational modification including γ-carboxylation. The precursor FIX protein has the following parts, starting with a signal peptide at the amino-terminal end, which directs the protein to the endoplasmic reticulum (ER), and continuing with the 18 residue length propeptide. The propeptide contains the γ‐carboxylase recognition site (γ‐CRS) in the N‐terminal part of the propeptide for connecting substrate to the gamma-glutamyl carboxylase (GGCX), and a peptidase recognition site in the C‐terminal for cleaving by PACE/furin (Higgins‐Gruber SL et al. 2010). GGCX in the ER γ-carboxylates twelve glutamate residues on F9(29-461) (pro-factor IX) converting the specific glutamic acids in the GLA domain to γ‐carboxyglutamic acid residues. MK4 (vitamin K hydroquinone) is oxidized to MK4 epoxide in the process (Berkner KL 2000; Furie B et al. 1999; Stenina O et al. 2001; Morris DP et al. 1995; Ware J et al. 1989). The signal peptide and the propeptide sequence of FIX are removed before the protein is secreted into the circulation,
Naturally occurring hemophilia B (HB)-associated point mutations in the FIX propeptide sequence such as F9 N43Q/L or F9 N46S reduce affinity to GGCX resulting in reduced γ-carboxylation and aberrant propeptide processing (Bentley AK et al. 1986; Rabiet MJ et al. 1987; Diuguid DL et al. 1986; Ware J et al. 1989; de la Salle C et al. 1993). FIX variants are secreted into the circulation with a mutant 18-amino acid propeptide still attached (Bentley AK et al. 1986; Galeffi P & Brownlee GG 1987). However, unprocessed FIX variants showed altered phospholipid binding properties and delayed activation by factor XIa (Liddell MB et al. 1989; Ware J et al. 1989; de la Salle C et al. 1993; Wojcik EG et al. 1997; Bristol JA et al. 1993, 1994). In addition, beta-hydroxylation of aspartic acid 110 (D110) is an independent process which does not require vitamin K and is mediated through a hydroxylation recognition site in the mature Factor IX, not in the propeptide (Rabiet MJ et al. 1987).
The Reactome event describes defective γ-carboxylation of FIX variants due to HB-associated genetic defects in the propeptide sequence of the F9 gene.
Reaction - small molecule participants:
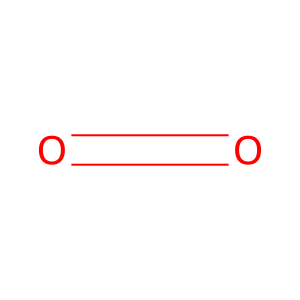
O2 [endoplasmic reticulum lumen]
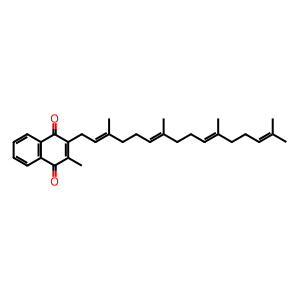
MK4 [endoplasmic reticulum lumen]
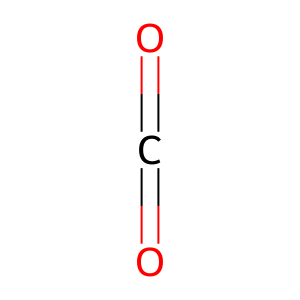
CO2 [endoplasmic reticulum lumen]
======
Reaction input - small molecules:
dioxygen
menaquinone-4
carbon dioxide
Reaction output - small molecules: