Reaction: Disulfiram covalently modifies Cys191 in GSDMD
- in pathway: Pyroptosis
During inflammation, the inflammatory caspase‑1 (CASP1) can be activated downstream of canonical inflammasome activation in response to sensing of pathogen‑derived particles or host‑derived danger signals (reviewed in Kelley N et al. 2019; Zheng D et al. 2020). The non‑canonical inflammasome assembly is mediated by CASP4, CASP5 in humans and CASP11 in mice upon sensing intracellular bacterial lipopolysaccharide (LPS) (Vigano E et al. 2015; Kayagaki N et al. 2015). Activated inflammatory caspases induce a proinflammatory cell death known as pyroptosis via the proteolytic processing of gasdermin D (GSDMD) (Shi J et al. 2015; Kayagaki N et al. 2015; He W et al. 2015; Ding J et al. 2016; Liu X et al. 2016; Sborgi L et al. EMBO J 2016). Intact GSDMD cannot form pores due to the inhibitory function of its C-terminal domain. Caspase‑mediated cleavage of GSDMD releases the C‑terminal fragment of GSDMD (276‑484) (Shi J et al. 2015), enabling the N‑terminal fragment of GSDMD (1‑275) to form pores in cellular membranes leading to cytokine release and pyroptosis (Ding J et al. 2016; Liu X et al. 2016; Sborgi L et al. 2016; Mulvihill E et al. 2018). Disulfiram, the thiol‑reactive drug also known as antabuse, was found to inhibit nigericin‑induced NLRP3‑mediated pyroptosis and inflammatory cytokine release in LPS‑primed human monocytic THP‑1 cells (Hu JJ et al. 2020). Similar results were obtained for the non‑canonical (caspase‑11‑dependent) mouse inflammasome pathway induced by LPS electroporation in mouse immortalized bone marrow‑derived macrophages (iBMDMs) (Hu JJ et al. 2020). Nano‑liquid chromatography‑tandem mass spectrometry (nano‑LC‑MS/MS) identified a dithiodiethylcarbamoyl adduct of Cys191 in human GSDMD suggesting that disulfiram covalently modified Cys191 of GSDMD. The importance of Cys191 of GSDMD for disulfiram activity was further confirmed by a site‑directed mutational analysis (Hu JJ et al. 2020). In line with these findings, necrosulfonamide (NSA) was identified as a potent inhibitor of pyroptosis by targeting GSDMD at Cys191 (Rathkey JK et al. 2018), and dimethyl fumarate modifies Cys191 to form S-(2-succinyl)-cysteine and block pyroptosis (Humphries F et al. 2020). Cys191 in human GSDMD (corresponding to Cys192 in mouse) is thought to be critical for the GSDMD oligomerization and pore formation (reviewed in Pandeya A et al. 2019). Further, disulfiram allowed cleavage of pro‑interleukin 1β (IL‑1β) and GSDMD, but abrogated GSDMD pore formation and blocked IL‑1β release in human and mouse cells (Hu JJ et al. 2020). Moreover, GSDMD‑mediated pyroptosis when overactivated can lead to sepsis. Elevated levels of GSDMD were noted in microparticles isolated from plasma of septic patients (Homsy E et al. 2019). In the murine sepsis model, GSDMD‑deficient mice showed significantly improved survival compared to the wild type mice (Kambara H et al. 2018). Disulfiram activity protected mice from LPS‑induced septic shock (Hu JJ et al. 2020). The data suggest that disulfiram blocks GSDMD pore formation and pyroptosis by modifying Cys191 of GSDMD and point to the possibility of using disulfiram to counteract human diseases due to excessive inflammation (Hu JJ et al. 2020).
Reaction - small molecule participants:
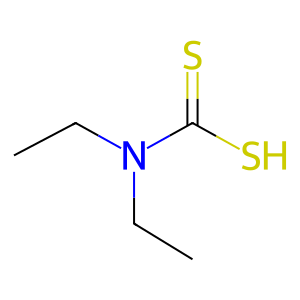
diethyldithiocarbamic acid [cytosol]
Reactome.org reaction link: R-HSA-9693324
======
Reaction input - small molecules:
Reaction output - small molecules:
diethyldithiocarbamic acid
Reactome.org link: R-HSA-9693324