Reaction: PTPRZ dephosphorylates ligand-bound ALK dimers
- in pathway: Signaling by ALK
ALK is dephosphorylated by PTPRZ leading to loss of activity (Perez-Pinera et al, 2007).
Some studies suggest that, rather than being canonically activated by direct binding of PTN and MDK, ALK is activated in a ligand-independent manner by the binding of these ligands to PTPRZ (and possibly PTPRB) instead. PTN- and MDK-binding to PTPRZ/B promotes their oligomerization and catalytic inactivation, thus inhibiting the phosphatase activity and allowing the autoactivation of ALK to predominate (Perez-Pinera et al, 2007; Fukada et al, 2006; Maeda et al, 1999; Kuboyama et al, 2016; reviewed in Deuel, 2013). Discrepancies between these two models remain to be worked out.
Some studies suggest that, rather than being canonically activated by direct binding of PTN and MDK, ALK is activated in a ligand-independent manner by the binding of these ligands to PTPRZ (and possibly PTPRB) instead. PTN- and MDK-binding to PTPRZ/B promotes their oligomerization and catalytic inactivation, thus inhibiting the phosphatase activity and allowing the autoactivation of ALK to predominate (Perez-Pinera et al, 2007; Fukada et al, 2006; Maeda et al, 1999; Kuboyama et al, 2016; reviewed in Deuel, 2013). Discrepancies between these two models remain to be worked out.
Reaction - small molecule participants:
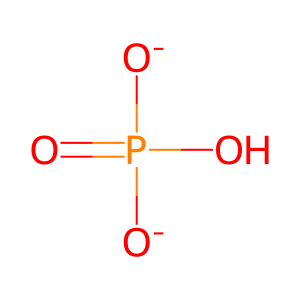
Pi [cytosol]
H2O [cytosol]
Reactome.org reaction link: R-HSA-9700200
======
Reaction input - small molecules:
water
Reaction output - small molecules:
hydrogenphosphate
Reactome.org link: R-HSA-9700200