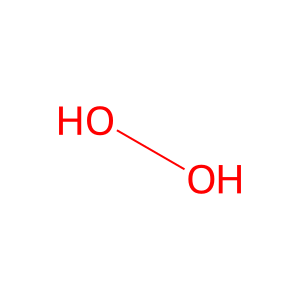
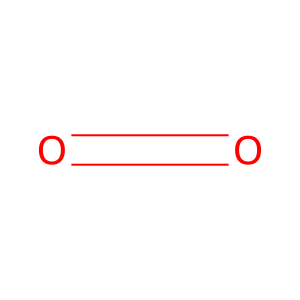
Reaction: H2O2 reduces MetHb
- in pathway: Cytoprotection by HMOX1
Hydrogen peroxide (H2O2) can reduce methemoglobin (metHb) but this is a slow reaction. However, the fast and practically irreversible reaction of ferrous heme-iron in ferrohemoglobin with carbon monoxide (CO) results in a shift of the equilibrium towards the carboxy forms of hemoglobin. The main impact of CO is not reduction of ferric heme-iron per se, but rather its arrest in the ferrous carboxy complex, a practically irreversible process. Equilibrium is then shifted via Le Chatelier's principle. Therefore, the net result of the reaction appears to be replacement of injurious plasma components, metHb and H2O2, by physiological, harmless, metabolites (Sher et al, 2012).
Reaction - small molecule participants:
H2O [extracellular region]
O2 [extracellular region]
H2O2 [extracellular region]
Reactome.org reaction link: R-HSA-9709360
======
Reaction input - small molecules:
hydrogen peroxide
Reaction output - small molecules:
water
dioxygen
Reactome.org link: R-HSA-9709360