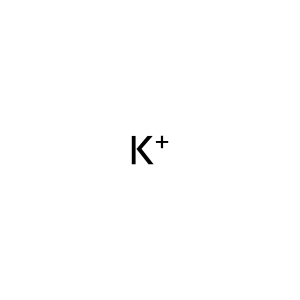Reaction: SARS-CoV-1 3a tetramer induces potassium efflux
SARS-CoV-1 viroporin 3a is a membrane protein that can translocate to the plasma membrane and, via shedding, to other cells. The cytoplasmic domain of 3a contains the tyrosine based sorting motif (YXXΦ, where X can be any residue and Φ is a residue with a bulky hydrophobic side chain) which is responsible for Golgi to plasma membrane transport (Minakshi R & Padhan K 2014). In the membranes, 3a forms a homotetrameric inward-rectifying potassium ion channel which is inhibited by barium ions and 4-aminopyridine (Chan CM et al. 2009; Chien TH et al. 2013). The ion channel activity of 3a is linked to SARS-CoV-1-induced cell death in various mammalian cells (Law PTW et al. 2005; Freundt EC et al. 2010; Chan CM et al. 2009; Chien TH et al. 2013; Chen IY et al. 2019; Yue Y et al. 2018). Blocking potassium efflux from the cell impaired the ability of viral 3a protein to induce the caspase-dependent extrinsic apoptotic signalling pathway (Chan CM et al, 2009; Chien TH et al, 2013). In addition, membrane-bound 3a oligomers were found to activate the NLRP3 inflammasome and interleukin 1 beta (IL-1β) secretion via potassium efflux causing necrotic cell death (Chen IY et al. 2019; Yue Y et al. 2018). These data suggest that the ion channel activity of SARS-CoV-1 3a oligomers regulates both non-lytic apoptosis and highly inflammatory pyroptosis.Further, mutagenesis studies revealed that membrane association (Ren Y et al. 2020) and ion channel activities (Yue Y et al. 2018) of SARS-CoV-1 3a are involved but not critical for triggering cell death suggesting that 3a can also induce apoptotic or pyroptotic cell death in a membrane-independent manner (Ren Y et al. 2020).