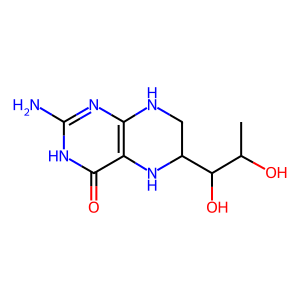
Reaction: The cofactor BH4 is required for electron transfer in the eNOS catalytic cycle
- in pathway: eNOS activation
The cofactor tetrahydrobiopterin (BH4) ensures endothelial nitric oxide synthase (eNOS) couples electron transfer to L-arginine oxidation (Berka et al. 2004). During catalysis, electrons derived from NADPH transfer to the flavins FAD and FMN in the reductase domain of eNOS and then on to the ferric heme in the oxygenase domain of eNOS. BH4 can donate an electron to intermediates in this electron transfer and is oxidised in the process, forming the BH3 radical. This radical can be reduced back to BH4 by iron, completing the cycle and forming ferrous iron again. Heme reduction enables O2 binding and L-arginine oxidation to occur within the oxygenase domain (Stuehr et al. 2009).
Reaction - small molecule participants:
BH4 [cytosol]
BH4 [cytosol]
BH4 [cytosol]
Reactome.org reaction link: R-HSA-1497784
======
Reaction input - small molecules:
5,6,7,8-tetrahydrobiopterin
5,6,7,8-tetrahydrobiopterin
5,6,7,8-tetrahydrobiopterin
Reaction output - small molecules:
Reactome.org link: R-HSA-1497784