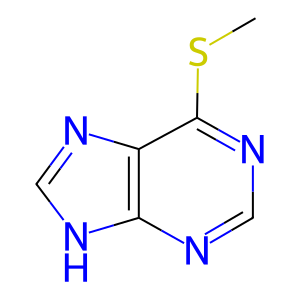
Reaction: TPMT transfers CH3 from AdoMet to 6MP
- in pathway: Methylation
Methylation is the major biotransformation route of thiopurine drugs such as 6-mercaptopurine (6MP), used in the treatment of inflammatory diseases such as rheumatoid arthritis and childhood acute lymphoblastic leukemia. 6MP and its thioguanine nucleotide metabolites are principally inactivated by thiopurine methyltransferase (TPMT)-catalysed S-methylation.
Defects in TPMT can cause thiopurine S methyltransferase deficiency (TPMT deficiency; MIM:610460). Patients with intermediate or no TPMT activity are at risk of toxicity such as myelosuppression after receiving standard doses of thiopurine drugs. Inter individual differences in response to these drugs are largely determined by genetic variation at the TPMT locus. TPMT exhibits an autosomal co dominant phenotype: About one in 300 people in Caucasian, African, African-American, and Asian populations are TPMT deficient. Approximately 6-10% of people in these populations inherit intermediate TPMT activity and are heterozygous at the TPMT locus. The rest are homozygous for the wild type allele and have high levels of TPMT activity. (Remy 1963, Weinshilboum et al. 1999, Couldhard & Hogarth 2005, Al Hadithy et al. 2005, Azimi et al. 2014).
Defects in TPMT can cause thiopurine S methyltransferase deficiency (TPMT deficiency; MIM:610460). Patients with intermediate or no TPMT activity are at risk of toxicity such as myelosuppression after receiving standard doses of thiopurine drugs. Inter individual differences in response to these drugs are largely determined by genetic variation at the TPMT locus. TPMT exhibits an autosomal co dominant phenotype: About one in 300 people in Caucasian, African, African-American, and Asian populations are TPMT deficient. Approximately 6-10% of people in these populations inherit intermediate TPMT activity and are heterozygous at the TPMT locus. The rest are homozygous for the wild type allele and have high levels of TPMT activity. (Remy 1963, Weinshilboum et al. 1999, Couldhard & Hogarth 2005, Al Hadithy et al. 2005, Azimi et al. 2014).
Reaction - small molecule participants:
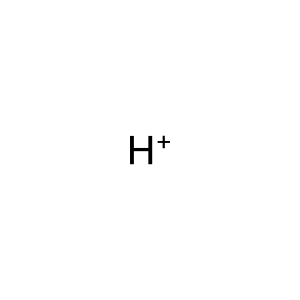
H+ [cytosol]
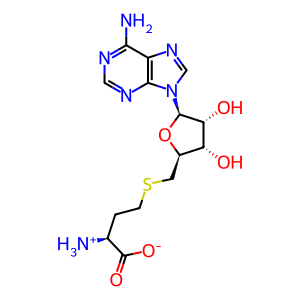
AdoHcy [cytosol]
6MMP [cytosol]
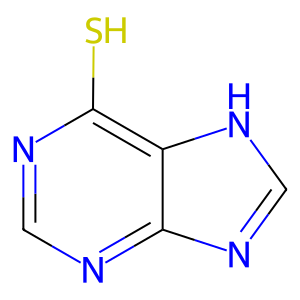
6MP [cytosol]
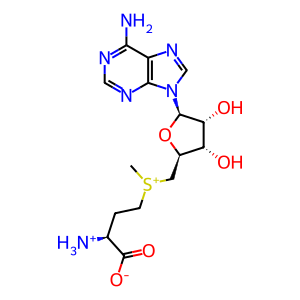
AdoMet [cytosol]
Reactome.org reaction link: R-HSA-158609
======
Reaction input - small molecules:
purine-6-thiol
S-adenosyl-L-methionine zwitterion
Reaction output - small molecules:
hydron
S-adenosyl-L-homocysteine zwitterion
6-methylthiopurine
Reactome.org link: R-HSA-158609