Molecule: S-adenosyl-L-homocysteine zwitterion
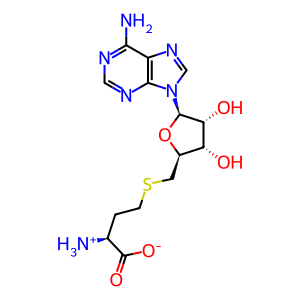
Zwitterionic form of S-adenosyl-L-homocysteine arising from migration of a proton from the carboxy group to the alpha-amino group; major species at pH 7.3.
Synonyms for S-adenosyl-L-homocysteine zwitterion :
S-adenosyl-L-homocysteine
Molecular Formula: C14H20N6O5S
Molecular wt: 384.41100 g/mole
Charge: 0
SMILES: Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H]([NH3+])C([O-])=O)[C@@H](O)[C@H]1O
InChIKey: ZJUKTBDSGOFHSH-WFMPWKQPSA-N
InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1
Sample reactions for this molecule:
