Reaction: Activated FGFR1 mutants and fusions bind PLCG1
- in pathway: Signaling by FGFR1 in disease
Although it has not been rigourously established, there is some evidence that PLC-gamma signaling may be activated after autophosphorylation of some FGFR mutants, analagous to the wild type receptor (see for instance, Hart, 2000; Chen, 2005; Cha, 2008; di Martino, 2009; Gartside, 2009; Cross, 2000; Hatch, 2006). The extent to which each of the mutants activates this pathway and to which proliferation and tumorigenesis relies on PLC-gamma dependent signaling, remains to be more firmly established. FGFR1 fusions with ZMYM2, BCR, FGFR1OP and TRIM24 all result in recruitment and phosphorylation of PLCgamma, and where mutational studies have been performed, mutation of the PLCgamma binding site Y766 has been shown to abrogate this signaling (Guasch, 2001; Roumiantsev, 2004, Lelievre, 2008, Chase, 2007). In the case of BCR-FGFR1 and ZMYM2-FGFR1, mutation of the PLCgamma binding site significantly decreased the transformative phenotype of the FGFR1 fusion (Roumiantsev, 2004).
Reaction - small molecule participants:
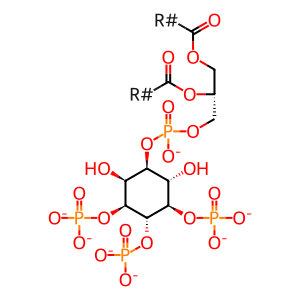
PI(3,4,5)P3 [plasma membrane]
Reactome.org reaction link: R-HSA-1839094
======
Reaction input - small molecules:
1-phosphatidyl-1D-myo-inositol 3,4,5-trisphosphate(7-)
Reaction output - small molecules:
Reactome.org link: R-HSA-1839094