Reaction: ALAD condenses 2 dALAs to form PBG
- in pathway: Heme biosynthesis
5-Aminolevulinic acid dehydratase (ALAD aka porphobilinogen synthase, PBGS), catalyzes the asymmetric condensation of two molecules of ALA to form porphobilinogen (PBG). The substrate that becomes the acetyl side chain-containing half of PBG is called A-side ALA; the half that becomes the propionyl side chains and the pyrrole nitrogen is called P-ALA (Jaffe 2004). PBG is the first pyrrole formed, the precursor to all tetrapyrrole pigments such as heme and chlorophyll. There are at least eight bonds that are made or broken during this reaction. The active form of the ALAD enzyme is an octamer complexed with eight Zn2+ ions, four that are strongly bound and four that are weakly bound. The four weakly bound ones are dispensible for enzyme activity in vitro (Bevan et al. 1980; Mitchell et al. 2001).
Deficiencies of ALAD enzyme in vivo are associated with 5-aminolevulinate dehydratase-deficient porphyria (e.g., Akagi et al. 2000).
Deficiencies of ALAD enzyme in vivo are associated with 5-aminolevulinate dehydratase-deficient porphyria (e.g., Akagi et al. 2000).
Reaction - small molecule participants:
H2O [cytosol]
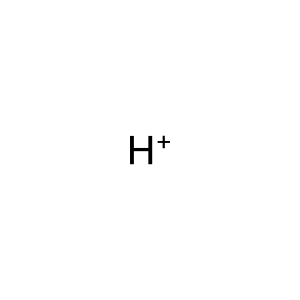
H+ [cytosol]
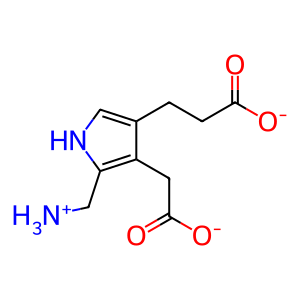
PBG [cytosol]
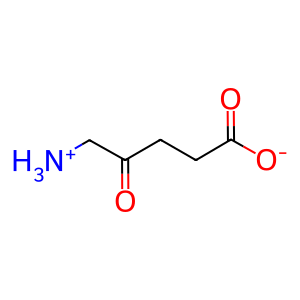
dALA [cytosol]
Reactome.org reaction link: R-HSA-189439
======
Reaction input - small molecules:
5-ammoniolevulinate
Reaction output - small molecules:
water
hydron
porphobilinogen(1-)
Reactome.org link: R-HSA-189439