Reaction: Procollagen lysyl hydroxylases convert collagen lysines to 5-hydroxylysines
- in pathway: Collagen biosynthesis and modifying enzymes
Lysyl hydroxylase (LH) (E.C. 1.14.11.4) is a dimeric enzyme that catalyzes the formation of (2S,5R)-5-hydroxylysyl residues (5-Hyl) in proteins (reviewed in Myllyharju & Kivirikko 2001) within a peptide linkage at the Y position of the repeating X-Y-Gly sequence motif. The extent of 5-Hyl formation is much more variable than that of hydroxyproline. It varies between collagen types, tissues and by physiological state (Miller 1984). 5-Hyl content also differs between the helical and telopeptide domains. This and the observation that purified lysyl hydroxylase failed to hydroxylate Lys in the telopeptide domains has led to speculation that there are separate enzymes responsible for Lys hydroxylation in the helical and telopeptide domains (Royce & Barnes 1985, Gerriets et al. 1993). The LH2b isoform may be the telopeptide-specific form (Pornprasertsuk et al. 2004).
In human type I collagen, there are 38 residues of Lys in each alpha-1 chain (36 in the helical domain, 1 each in the C- and N-telopeptide domains) and 31 in each alpha-2 chain (30 in the helical domain,1 in the N-telopeptide and none in the C-telopeptide domains) (Yamauchi & Shiiba 2002).
LH requires ferrous iron, oxygen, 2-oxoglutarate, and ascorbate. The hydroxylation reaction occurs during collagen biosynthesis in the ER as a co- and post-translational event, before triple helix formation. Three LH isoforms have been characterized in humans, encoded by the genes PLOD1-3. The isoforms appear to have preferences for certain collagen types, e.g. LH3 preferentially binds collagen types IV, VI, XI and XII (Myllyla et al. 2007). LH3 has galactosyltransferase and glucosyltransferase activities in addition to its lysyl hydroxylase activity (Heikkinen et al. 2000, Wang et al. 2002), a multifunctionality also seen in the single C. elegans orthologue (Wang et al. 2002a, b). Hydroxylysine residues can form stable intermolecular cross-links between collagen molecules in fibrils and also represent sites for glucosyl- and galactosyl- carbohydrate attachment.
In this reaction all collagen subtypes are represented as having a single hydroxylysine.
In human type I collagen, there are 38 residues of Lys in each alpha-1 chain (36 in the helical domain, 1 each in the C- and N-telopeptide domains) and 31 in each alpha-2 chain (30 in the helical domain,1 in the N-telopeptide and none in the C-telopeptide domains) (Yamauchi & Shiiba 2002).
LH requires ferrous iron, oxygen, 2-oxoglutarate, and ascorbate. The hydroxylation reaction occurs during collagen biosynthesis in the ER as a co- and post-translational event, before triple helix formation. Three LH isoforms have been characterized in humans, encoded by the genes PLOD1-3. The isoforms appear to have preferences for certain collagen types, e.g. LH3 preferentially binds collagen types IV, VI, XI and XII (Myllyla et al. 2007). LH3 has galactosyltransferase and glucosyltransferase activities in addition to its lysyl hydroxylase activity (Heikkinen et al. 2000, Wang et al. 2002), a multifunctionality also seen in the single C. elegans orthologue (Wang et al. 2002a, b). Hydroxylysine residues can form stable intermolecular cross-links between collagen molecules in fibrils and also represent sites for glucosyl- and galactosyl- carbohydrate attachment.
In this reaction all collagen subtypes are represented as having a single hydroxylysine.
Reaction - small molecule participants:
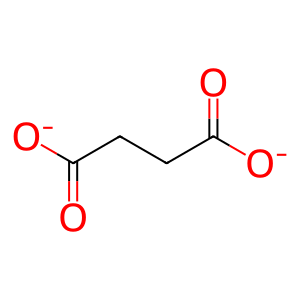
SUCCA [endoplasmic reticulum lumen]
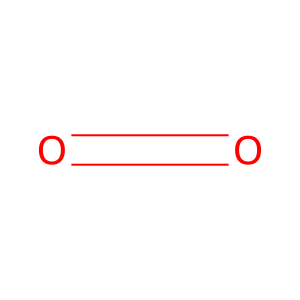
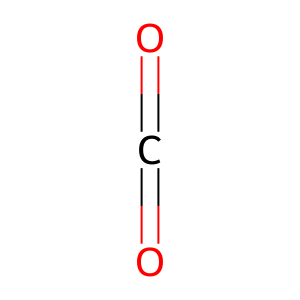
CO2 [endoplasmic reticulum lumen]
O2 [endoplasmic reticulum lumen]
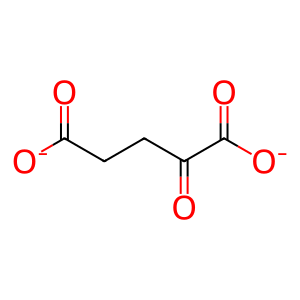
2OG [endoplasmic reticulum lumen]
Reactome.org reaction link: R-HSA-1981104
======
Reaction input - small molecules:
dioxygen
2-oxoglutarate(2-)
Reaction output - small molecules:
succinate(2-)
carbon dioxide
Reactome.org link: R-HSA-1981104