Reaction: PI3K gain of function mutants phosphorylate PIP2 to PIP3
- in pathway: Constitutive Signaling by Aberrant PI3K in Cancer
Constitutively active PI3K complex produces PIP3 in the absence of growth stimuli, resulting in aberrant activation of downstream AKT signaling that positively regulates cell growth and survival. The PIK3CA gene, encoding the catalytic subunit of PI3K (p110alpha), is one of the most frequently mutated oncogenes in cancer. Hotspot mutations are found in the helical domain and kinase domain of PIK3CA, with the most frequent mutations being E545K substitution in the helical domain and H1047R substitution in the kinase domain.
The oncogenic PIK3CA mutants annotated here preserve their ability to bind PIK3R1 (p85alpha) regulatory subunit, but are constitutively active either because the inhibitory interactions with PIK3R1 are relieved, or because the conformation of the catalytic domain is changed. Missense mutations that result in substitution of amino acids at positions 542, 545 or 546 of PI3K disrupt an inhibitory interaction between the helical domain of PIK3CA and the nSH2 domain of PIK3R1. The effect of substitution of glutamic acid residue at position 545 has been studied in detail in PIK3CA E545K mutant, where glutamic acid is replaced with lysine (Miled et al. 2007, Huang et al. 2007, Zhao et al. 2005). The gain-of-function has been experimentally confirmed for PIK3CA E545A mutant (Horn et al. 2008), while PIK3CA E545G, PIK3CA E545Q and PIK3CA E545V mutants are assumed to behave similarly. The structural and functional consequences of glutamic acid to lysine substitution at position 542, in PIK3CA E542K mutant, have been established (Miled et al. 2007, Horn et al. 2008) and are extrapolated to PIK3CA E542Q and PIK3CA E542V mutants. A less frequent substitution of glutamine residue at position 546 follows the same mechanism, as shown for PIK3CA Q546K mutant (Miled et al. 2007) and extrapolated to PIK3CA Q546E, PIK3CA Q546H, PIK3CA Q546L, PIK3CA Q546P and PIK3CA Q546R mutants.
In the kinase domain of PIK3CA, substitution of histidine residue at position 1047 or methionine residue at position 1043, detected in PIK3CA H1047R, PIK3CA H1047L, PIK3CA H1047Y, PIK3CA M1043I, PIK3CA M1043T and PIK3CA M1043V mutants, is predicted to change the conformation of the activation loop (Huang et al. 2007) and was shown to confer constitutive activity, in the absence of growth factors, to PIK3CA H1047R, PIK3CA H1047L and PIK3CA M1043I mutants (Zhao et al. 2005, Horn et al. 2008). The catalytic activity of PIK3CA H1047R, PIK3CA H1047L and PIK3CA M1043I mutants may be further increased by binding of PIK3R1 regulatory subunit to phosphopeptides generated by activated receptor tyrosine kinases (Hon et al. 2011). PIK3CA H1047Y, PIK3CA M1043T and PIK3CA M1043V mutants are expected to behave similarly.
The arginine residue at position 38 of PIK3CA (R38) is located at a contact site between the ABD and kinase domains of PIK3CA. Substitution of this arginine residue with histidine in PIK3CA R38H mutant is likely to disrupt the interaction between the ABD domain and the kinase domain, causing a conformational change of the kinase domain that leads to increased enzymatic activity (Huang et al. 2007). PIK3CA R38H mutant shows reduced PIK3R1 binding and modestly increased catalytic activity (measured indirectly, via AKT1 phosphorylation) under serum starved conditions (Zhao et al. 2005). PIK3CA R38C, PIK3CA R38G and PIK3CA R38S mutants are expected to behave similarly.
Mutations in other conserved domains of PIK3CA, such as membrane-binding C2 domain (Mandelker et al. 2009), have not been annotated as their mechanism of action needs to be further elucidated.
Although less common than mutations in PIK3CA, mutations in PIK3R1, encoding the regulatory subunit of PI3K (p85alpha) have been recently described. Mutations mapping to iSH2 and nSH2 domains, the two domains of PIK3R1 involved in the inhibition of PIK3CA, which were shown to result in constitutive activity of PIK3R1 complex, are annotated here. An experimentally studied nSH2 domain mutant is PIK3R1 G376R (Sun et al. 2010). PIK3R1 iSH2 domain mutants, affected by amino acid substitutions and small inframe deletions, PIK3R1 D560Y (Jaiswal et al. 2009), PIK3R1 N564D (Jaiswal et al. 2009), PIK3R1 N564K (Sun et al. 2010), PIK3R1 H450_E451del (Urick et al. 2011), PIK3R1 K459del (Urick et al. 2011), PIK3R1 R574_T576del (Urick et al. 2011) and PIK3R1 Y463_L466del (Urick et al. 2011), were all shown to bind PIK3CA and confer constitutive activity to PI3K complex. PIK3R1 D560H, PIK3R1 R574I and PIK3R1 R574T mutants are expected to behave similarly to functionally characterized D560 and R574 substitution mutants.
Co-occurrence of PIK3CA and PIK3R1 mutations has been documented in some tumors, but since it is rare and the exact clinical combinations of PIK3CA and PIK3R1 mutants have not been studied, complexes of PIK3CA mutants with PIK3R1 mutants are not shown (Urick et al. 2011).
Although rare, perturbations in genes encoding other isoforms of PI3K subunits have also been reported in cancers. Mutations in PIK3R2, encoding PI3K regulatory subunit isoform p85beta, are found infrequently in endometrial cancers, but have not been functionally studied (Cheung et al. 2011). They are not shown in this context. PIK3CB, encoding PI3K catalytic subunit isoform p110beta, can be overexpressed in cancer, mainly due to genomic gain. Several studies have shown that PTEN deficient cancer cell lines depend on PIK3CB (p110beta) for AKT activation and sustained growth (Wee et al. 2008, Jiang et al. 2010, Chen et al. 2011). PIK3CB activation synergizes with PTEN loss in mouse prostate cancer model (Jia et al. 2008). Mutations in PIK3CB are very rare, have not been functionally studied, and are therefore not shown. Structural studies indicate that, in comparison with PIK3CA (p110alpha), PIK3CB (p110beta) and PIK3CD (p110delta) form additional inhibitory contacts with the regulatory subunit p85alpha, and are therefore probably less prone to mutational activation (Burke et al. 2011).
For more information, please refer to recent reviews by Liu et al. 2009 and Vogt et al. 2009.
The oncogenic PIK3CA mutants annotated here preserve their ability to bind PIK3R1 (p85alpha) regulatory subunit, but are constitutively active either because the inhibitory interactions with PIK3R1 are relieved, or because the conformation of the catalytic domain is changed. Missense mutations that result in substitution of amino acids at positions 542, 545 or 546 of PI3K disrupt an inhibitory interaction between the helical domain of PIK3CA and the nSH2 domain of PIK3R1. The effect of substitution of glutamic acid residue at position 545 has been studied in detail in PIK3CA E545K mutant, where glutamic acid is replaced with lysine (Miled et al. 2007, Huang et al. 2007, Zhao et al. 2005). The gain-of-function has been experimentally confirmed for PIK3CA E545A mutant (Horn et al. 2008), while PIK3CA E545G, PIK3CA E545Q and PIK3CA E545V mutants are assumed to behave similarly. The structural and functional consequences of glutamic acid to lysine substitution at position 542, in PIK3CA E542K mutant, have been established (Miled et al. 2007, Horn et al. 2008) and are extrapolated to PIK3CA E542Q and PIK3CA E542V mutants. A less frequent substitution of glutamine residue at position 546 follows the same mechanism, as shown for PIK3CA Q546K mutant (Miled et al. 2007) and extrapolated to PIK3CA Q546E, PIK3CA Q546H, PIK3CA Q546L, PIK3CA Q546P and PIK3CA Q546R mutants.
In the kinase domain of PIK3CA, substitution of histidine residue at position 1047 or methionine residue at position 1043, detected in PIK3CA H1047R, PIK3CA H1047L, PIK3CA H1047Y, PIK3CA M1043I, PIK3CA M1043T and PIK3CA M1043V mutants, is predicted to change the conformation of the activation loop (Huang et al. 2007) and was shown to confer constitutive activity, in the absence of growth factors, to PIK3CA H1047R, PIK3CA H1047L and PIK3CA M1043I mutants (Zhao et al. 2005, Horn et al. 2008). The catalytic activity of PIK3CA H1047R, PIK3CA H1047L and PIK3CA M1043I mutants may be further increased by binding of PIK3R1 regulatory subunit to phosphopeptides generated by activated receptor tyrosine kinases (Hon et al. 2011). PIK3CA H1047Y, PIK3CA M1043T and PIK3CA M1043V mutants are expected to behave similarly.
The arginine residue at position 38 of PIK3CA (R38) is located at a contact site between the ABD and kinase domains of PIK3CA. Substitution of this arginine residue with histidine in PIK3CA R38H mutant is likely to disrupt the interaction between the ABD domain and the kinase domain, causing a conformational change of the kinase domain that leads to increased enzymatic activity (Huang et al. 2007). PIK3CA R38H mutant shows reduced PIK3R1 binding and modestly increased catalytic activity (measured indirectly, via AKT1 phosphorylation) under serum starved conditions (Zhao et al. 2005). PIK3CA R38C, PIK3CA R38G and PIK3CA R38S mutants are expected to behave similarly.
Mutations in other conserved domains of PIK3CA, such as membrane-binding C2 domain (Mandelker et al. 2009), have not been annotated as their mechanism of action needs to be further elucidated.
Although less common than mutations in PIK3CA, mutations in PIK3R1, encoding the regulatory subunit of PI3K (p85alpha) have been recently described. Mutations mapping to iSH2 and nSH2 domains, the two domains of PIK3R1 involved in the inhibition of PIK3CA, which were shown to result in constitutive activity of PIK3R1 complex, are annotated here. An experimentally studied nSH2 domain mutant is PIK3R1 G376R (Sun et al. 2010). PIK3R1 iSH2 domain mutants, affected by amino acid substitutions and small inframe deletions, PIK3R1 D560Y (Jaiswal et al. 2009), PIK3R1 N564D (Jaiswal et al. 2009), PIK3R1 N564K (Sun et al. 2010), PIK3R1 H450_E451del (Urick et al. 2011), PIK3R1 K459del (Urick et al. 2011), PIK3R1 R574_T576del (Urick et al. 2011) and PIK3R1 Y463_L466del (Urick et al. 2011), were all shown to bind PIK3CA and confer constitutive activity to PI3K complex. PIK3R1 D560H, PIK3R1 R574I and PIK3R1 R574T mutants are expected to behave similarly to functionally characterized D560 and R574 substitution mutants.
Co-occurrence of PIK3CA and PIK3R1 mutations has been documented in some tumors, but since it is rare and the exact clinical combinations of PIK3CA and PIK3R1 mutants have not been studied, complexes of PIK3CA mutants with PIK3R1 mutants are not shown (Urick et al. 2011).
Although rare, perturbations in genes encoding other isoforms of PI3K subunits have also been reported in cancers. Mutations in PIK3R2, encoding PI3K regulatory subunit isoform p85beta, are found infrequently in endometrial cancers, but have not been functionally studied (Cheung et al. 2011). They are not shown in this context. PIK3CB, encoding PI3K catalytic subunit isoform p110beta, can be overexpressed in cancer, mainly due to genomic gain. Several studies have shown that PTEN deficient cancer cell lines depend on PIK3CB (p110beta) for AKT activation and sustained growth (Wee et al. 2008, Jiang et al. 2010, Chen et al. 2011). PIK3CB activation synergizes with PTEN loss in mouse prostate cancer model (Jia et al. 2008). Mutations in PIK3CB are very rare, have not been functionally studied, and are therefore not shown. Structural studies indicate that, in comparison with PIK3CA (p110alpha), PIK3CB (p110beta) and PIK3CD (p110delta) form additional inhibitory contacts with the regulatory subunit p85alpha, and are therefore probably less prone to mutational activation (Burke et al. 2011).
For more information, please refer to recent reviews by Liu et al. 2009 and Vogt et al. 2009.
Reaction - small molecule participants:
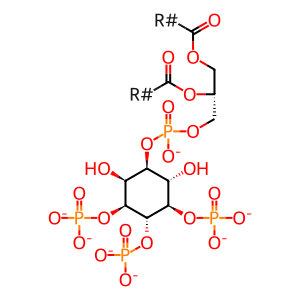
PI(3,4,5)P3 [plasma membrane]
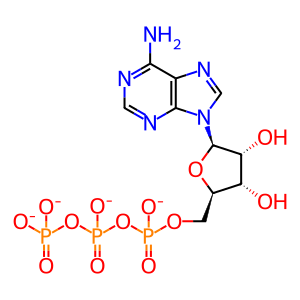
ADP [cytosol]
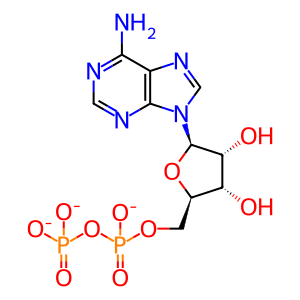
ATP [cytosol]
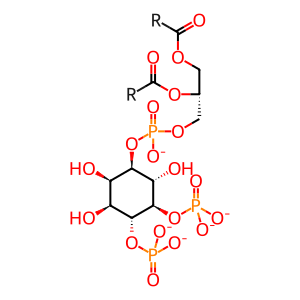
PI(4,5)P2 [plasma membrane]
Reactome.org reaction link: R-HSA-2394007
======
Reaction input - small molecules:
ATP(4-)
1-phosphatidyl-1D-myo-inositol 4,5-bisphosphate(5-)
Reaction output - small molecules:
1-phosphatidyl-1D-myo-inositol 3,4,5-trisphosphate(7-)
ADP(3-)
Reactome.org link: R-HSA-2394007