Reaction: 3-ketopristanoyl-CoA + CoASH => 4,8,12-trimethyltridecanoyl-CoA + propionyl-CoA
- in pathway: Beta-oxidation of pristanoyl-CoA
Peroxisomal SCPx (Non-specific lipid transfer protein; SCP2) catalyzes the reaction of 3-ketopristanoyl-CoA and CoASH to form 4,8,12-trimethyltridecanoyl-CoA and propionyl-CoA. Both intact SCPx and an SCPx fragment corresponding to approximately the 430 aminoterminal residues of the protein are catalytically active in vitro; the latter form may predominate in vivo. Consistent with the role of rat SCPx in the beta-oxidation of branched-chain fatty acids in vitro (Wanders et al. 1997) mutations in the human protein are associated with loss of SCP thiolase activity in cell extracts in vitro, elevated levels of pristanic acid in the blood in vivo and the development of neurological defects (Ferdinandusse et al. 2006).
Reaction - small molecule participants:
propionyl CoA [peroxisomal matrix]
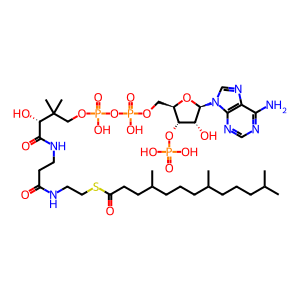
4,8,12-trimethyltridecanoyl-CoA [peroxisomal matrix]
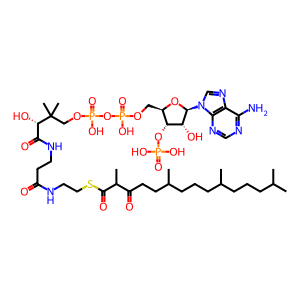
3-ketopristanoyl-CoA [peroxisomal matrix]
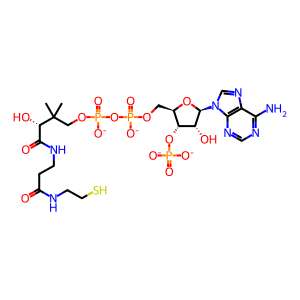
CoA-SH [peroxisomal matrix]
Reactome.org reaction link: R-HSA-390224
======
Reaction input - small molecules:
3-oxopristanoyl-CoA
coenzyme A(4-)
Reaction output - small molecules:
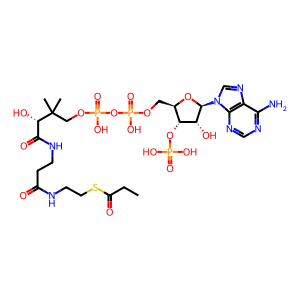
propionyl-CoA
4,8,12-trimethyltridecanoyl-CoA
Reactome.org link: R-HSA-390224