Reaction: CYGB dioxygenates NO
- in pathway: eNOS activation
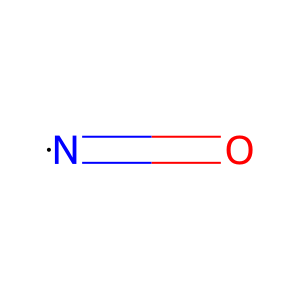
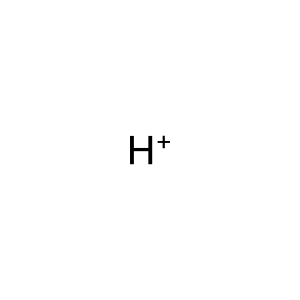
Vertebrates possess multiple respiratory globins that differ in structure, function, and tissue distribution. Three different globins have been described so far: haemoglobin facilitates oxygen transport in blood, myoglobin mediates oxygen transport and storage in the muscle and neuroglobin has a yet unidentified function in nerve cells. A fourth globin has been identified in mouse, human and zebrafish. It is ubiquitously expressed in human tissue and therefore called cytoglobin (CYGB) (Trent & Hargrove 2002). Unlike the specific expression patterns of Hb and Mb, CYGB is found in vascular smooth muscle, fibroblasts and cardiomyocytes. CYGB functions as a homodimer (Hamdane et al. 2003) and is localised to the cytosol. As well as oxygen binding capability, CYGB possesses nitric oxide dioxygenase activity (Halligan et al. 2009), a common feature amongst the globin family (Smagghe et al. 2008). CYGB consumes NO through the dioxygenase pathway, which regulates cell respiration and proliferation (Smagghe et al. 2008). O2 binds to the ferric form of CYGB (CYGB-Fe2+:O2). During NO dioxygenation, CYGB is reduced to the ferrous form (CYGB-Fe3+) (Gardner 2005).
Reaction - small molecule participants:
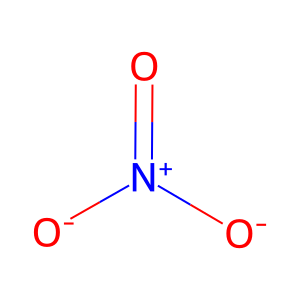
NO3- [cytosol]
NADP+ [cytosol]
NO [cytosol]
H+ [cytosol]
NADPH [cytosol]
Reactome.org reaction link: R-HSA-5340226
======
Reaction input - small molecules:
nitric oxide
hydron
NADPH(4-)
Reaction output - small molecules:
nitrate
NADP(3-)
Reactome.org link: R-HSA-5340226