Reaction: AKR1A1 oxidises BaPtDHD to BaP-7,8-dione
- in pathway: Glutathione conjugation
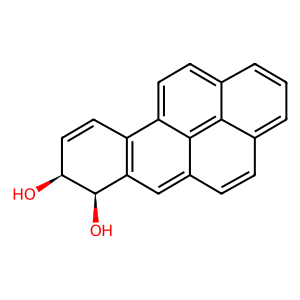
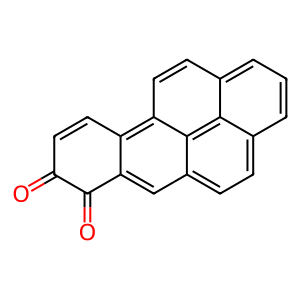
Polycyclic aromatic hydrocarbons (PAHs) are pro-carcinogens which require further metabolic activation to ellicit their harmful effects. Aldo-keto reductases (AKRs) such as alcohol dehydrogenase [NADP+] (AKR1A1) can catalyse the oxidation of proximate carcinogenic PAH trans-dihydrodiols to reactive and redox active PAH o-quinones. Redox-cycling of PAH o-quinones generate reactive oxygen species and subsequent oxidative DNA damage. The proximate PAH carcinogen benzo[a]pyrene-7,8-trans-dihydrodiol (BaPtDHD) is oxidised by AKR1A1 to yield BaP-7,8-catechol which is unstable and auto-oxidises to yield BaP-7,8-dione (Zhang et al. 2012).
Reaction - small molecule participants:
BaP-7,8-dione [cytosol]
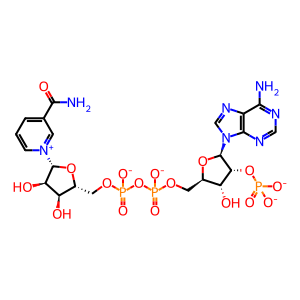
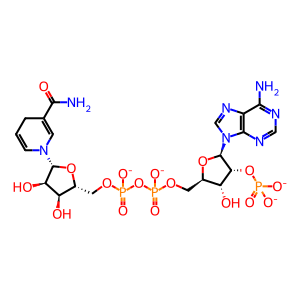
NADPH [cytosol]
NADP+ [cytosol]
BaPtDHD [cytosol]
Reactome.org reaction link: R-HSA-5692232
======
Reaction input - small molecules:
NADP(3-)
benzo[a]pyrene-cis-7,8-dihydrodiol
Reaction output - small molecules:
benzo[a]pyrene-7,8-dione
NADPH(4-)
Reactome.org link: R-HSA-5692232