Reaction: PRMT3 transfers 3xCH3 from 3xAdoMet to RPS2
- in pathway: RMTs methylate histone arginines
Protein arginine methyltransferase 3 (PRMT3) is a cytosolic enzyme that catalyzes the formation of omega-mono- or asymmetric dimethylarginine (Tang et al. 1998). It has a unique substrate binding N-terminal C2H2 Zn finger domain and a catalytic C-terminal domain that is homologous to other PRMTs (Zhang et al. 2000). PRMT3 associates with ribosomes in the cytosol, which contain its main in vivo substrate, the small ribosomal subunit Ribosomal protein S2 (RPS2). PRMT3 methylates arginines in the RG-rich N-terminal tail of RPS2 forming asymmetric dimethylarginines (Swiercz et al. 2005). Prmt3–null mice show developmental delay during embryogenesis and have embryos that are markedly smaller than wt , though size at birth is normal suggesting that PRMT3 loss can be compensated for in most cell types but may not be in under conditions that demand extremely fast protein synthesis (Swiercz et al. 2007).
Reaction - small molecule participants:
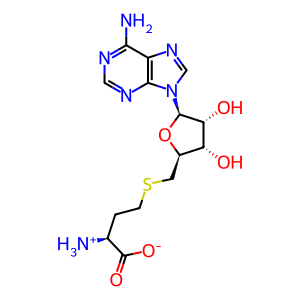
AdoHcy [cytosol]
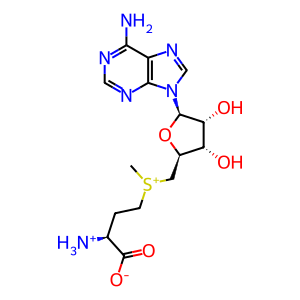
AdoMet [cytosol]
AdoHcy [cytosol]
AdoMet [cytosol]
Reactome.org reaction link: R-HSA-8879123
======
Reaction input - small molecules:
S-adenosyl-L-methionine zwitterion
S-adenosyl-L-methionine zwitterion
Reaction output - small molecules:
S-adenosyl-L-homocysteine zwitterion
S-adenosyl-L-homocysteine zwitterion
Reactome.org link: R-HSA-8879123