Reaction: ALOX5 dehydrogenates 7,17-diHp-DPAn-3 to 7,8-epoxy,17-HDPAn-3
- in pathway: Biosynthesis of DPAn-3-derived protectins and resolvins
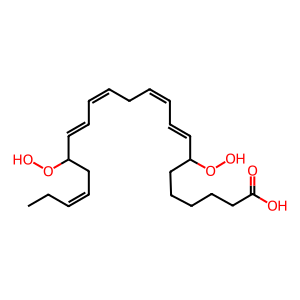
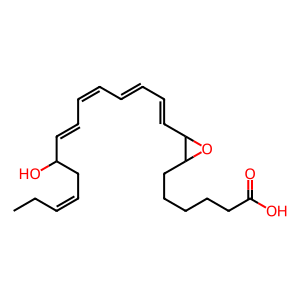
Instead of the dihydroperoxy intermediate being reduced, a lipoxygenase may mediate hydrogen abstraction from 7,17-dihydroperoxy-docosapentaenoic acid (7,17-diHp-DPAn-3) to form 7,8-epoxy, 17-hydroxydocosapentaenoic acid (7,8-epoxy,17-HDPAn-3) (Dalli et al. 2013). Although this is a proposed biosynthetic route for the formation of DPA-n-3 resolvins, it is assumed DPAn-3-derived SPMs follow a similar synthesis route to DHA- and EPA-derived SPMs and therefore, the lipoxygenase could be the dual-functional 5-lipoxygenase (ALOX5).
Reaction - small molecule participants:
7,8-epoxy,17-HDPAn-3 [cytosol]
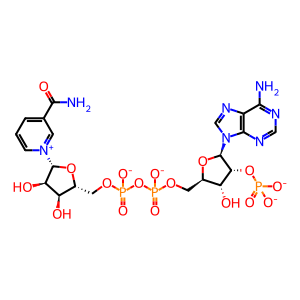
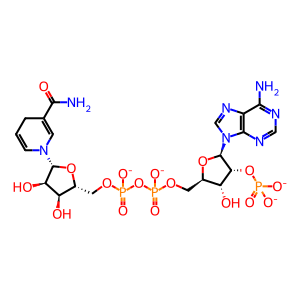
NADPH [cytosol]
7,17-diHp-DPAn-3 [cytosol]
NADP+ [cytosol]
Reactome.org reaction link: R-HSA-9025995
======
Reaction input - small molecules:
(8E,10Z,13Z,15E,19Z)-7,17-bis(hydroperoxy)docosapentaenoic acid
NADP(3-)
Reaction output - small molecules:
7,8-epoxy,17-hydroxy-(9E,11E,13Z,15E,19Z)-docosapentaenoic acid
NADPH(4-)
Reactome.org link: R-HSA-9025995