Reaction: Dehydrogenase dehydrogenates 17-HDHA to 17-oxo-DHA
- in pathway: Biosynthesis of electrophilic ω-3 PUFA oxo-derivatives
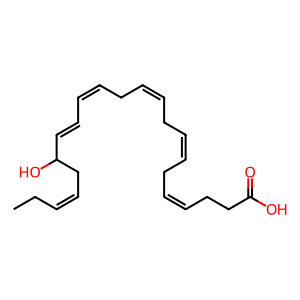
When acetylated by aspirin, dimeric acetylated-cyclooxygenase 2 (Ac-PTGS2 dimer aka COX2) in macrophages is able to form 17-hydroxy-docosahexaenoic acid (17-HDHA). This intermediate is then dehydrogenated by an unknown dehydrogenase to form the electrophilic oxo (EFOX) product 17-oxo-docosahexaenoic acid (17-oxo-DHA) (Groeger et al. 2010). Potential candidate enzymes are cellular dehydrogenases such as 3α-hydroxysteroid dehydrogenases (3α-HSDs), which can convert 13- and 17-HDHA into corresponding EFOXs in the presence of NAD(P)+ in vitro(supplementary data, Groeger et al. 2010) or 5- and 15-hydroxyeicosanoid dehydrogenases (5- and 15-HEDH), which convert LOX products to 5-and 15-oxoETE (Erlemann et al. 2007, Wendell et al. 2015). Anti-inflammatory actions of 17-oxo-DHA include acting as a peroxisome proliferator-activated receptor-γ (PPARγ) agonist to inhibit pro-inflammatory cytokine and nitric oxide production (Groeger et al. 2010, Cipollina et al. 2016). 17-oxo-DHA was also found to be a strong inducer of the anti-oxidant response, promoting Nrf2 nuclear accumulation, leading to the expression of heme oxygenase 1 and more than doubling glutathione levels (Cipollina et al. 2014).
Reaction - small molecule participants:
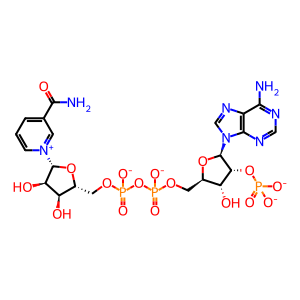
NADPH [cytosol]
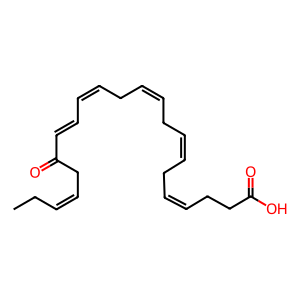
17-oxo-DHA [cytosol]
17-HDHA [cytosol]
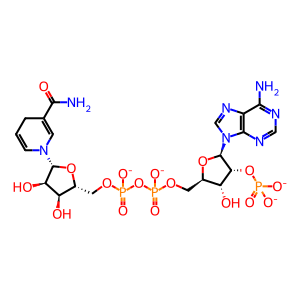
NADP+ [cytosol]
Reactome.org reaction link: R-HSA-9027562
======
Reaction input - small molecules:
(4Z,7Z,10Z,13Z,15E,19Z)-17-hydroxydocosahexaenoic acid
NADP(3-)
Reaction output - small molecules:
NADPH(4-)
(4Z,7Z,10Z,13Z,15E,19Z)-17-oxodocosahexaenoic acid
Reactome.org link: R-HSA-9027562