Reaction: TPMT transfers methyl group to 6MP, forming 6MeMP
- in pathway: Azathioprine ADME
6-mercaptopurine (6MP) can be metabolized via three competing pathways; oxidation by xanthine oxidase (XDH), methylation by thiopurine methyl S-transferase (TPMT) and conversion by hypoxanthine-guanine phosphoribosyltransferase (HPRT1).
6MP can be methylated to 6-methylmercaptopurine (6MeMP) by thiopurine methyltransferase (TPMT) (Weinshilboum et al. 1978). Although 6MeMP is an inactive metabolite, it is still capable of inhibiting purine biosynthesis (Hill & Bennett Jr 1980, Dervieux et al. 2001).
Genetic variability in TPMT enzyme activity is responsible for the majority of the individual differences observed in both the efficacy and toxicity of thiopurines. The TPMT gene exhibits more than 30 genetic polymorphisms that affect its enzymatic activity (Wang & Weinshilboum 2006, Colleoni et al. 2013). TPMT methylation competes with the activation pathway and influences the relative proportion of intracellular active 6-thioguanine nucleotides (6-TGNs) produced by a given individual. Patients with intermediate or absent TPMT activity can produce significantly higher concentrations of 6-TGNs, and can experience potentially life-threatening myelosuppression when treated with standard or even low dose therapy (Lennard & Lilleyman 1989, Lennard et al. 1989, Relling et al. 2011, Dean 2012).
6MP can be methylated to 6-methylmercaptopurine (6MeMP) by thiopurine methyltransferase (TPMT) (Weinshilboum et al. 1978). Although 6MeMP is an inactive metabolite, it is still capable of inhibiting purine biosynthesis (Hill & Bennett Jr 1980, Dervieux et al. 2001).
Genetic variability in TPMT enzyme activity is responsible for the majority of the individual differences observed in both the efficacy and toxicity of thiopurines. The TPMT gene exhibits more than 30 genetic polymorphisms that affect its enzymatic activity (Wang & Weinshilboum 2006, Colleoni et al. 2013). TPMT methylation competes with the activation pathway and influences the relative proportion of intracellular active 6-thioguanine nucleotides (6-TGNs) produced by a given individual. Patients with intermediate or absent TPMT activity can produce significantly higher concentrations of 6-TGNs, and can experience potentially life-threatening myelosuppression when treated with standard or even low dose therapy (Lennard & Lilleyman 1989, Lennard et al. 1989, Relling et al. 2011, Dean 2012).
Reaction - small molecule participants:
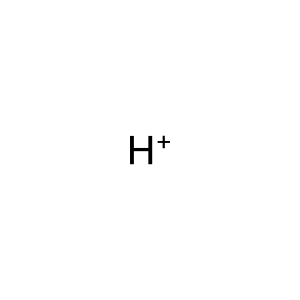
H+ [cytosol]
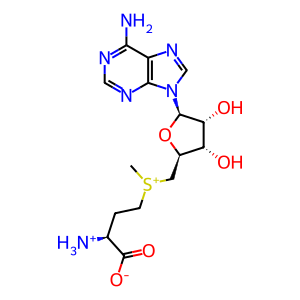
AdoHcy [cytosol]
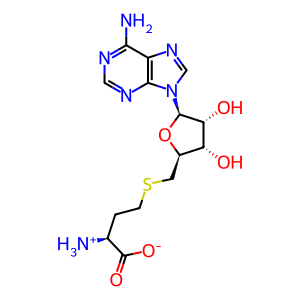
6MeMP [cytosol]
AdoMet [cytosol]
Reactome.org reaction link: R-HSA-9748983
======
Reaction input - small molecules:
S-adenosyl-L-methionine zwitterion
Reaction output - small molecules:
hydron
S-adenosyl-L-homocysteine zwitterion
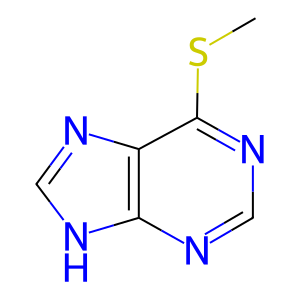
6-methylthiopurine
Reactome.org link: R-HSA-9748983