Reaction: SRXN1 reduces hyperoxidized PRDX1 dimer
- in pathway: NFE2L2 regulating anti-oxidant/detoxification enzymes
SRXN1 is a sulfiredoxin protein that reduces hyperoxidized members of the PRDX family in response to H2O2. PRDX dimers respond to the oxidative challenge of intracellular H2O2 through the two-step formation of an internal disulphide bond during the process of H2O2 reduction. This disulphide bond can be reduced by thioredoxin (TRX), regenerating the catalytically active PRDX dimer.
In some instances, the second step in this pathway is replaced by the hyperoxidation of the PRDX dimer to sulfinic acid, which is not reduceable by TRX. While this was previously thought to be irreversible, SRXN1 has been shown to reduce sulfinic acid in some PRDX family members, restoring the enzymatic activity (Chang et al, 2004; Woo et al, 2005).
In some instances, the second step in this pathway is replaced by the hyperoxidation of the PRDX dimer to sulfinic acid, which is not reduceable by TRX. While this was previously thought to be irreversible, SRXN1 has been shown to reduce sulfinic acid in some PRDX family members, restoring the enzymatic activity (Chang et al, 2004; Woo et al, 2005).
Reaction - small molecule participants:
PPi [cytosol]
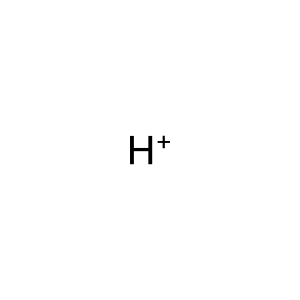
H+ [cytosol]
ADP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-9760094
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
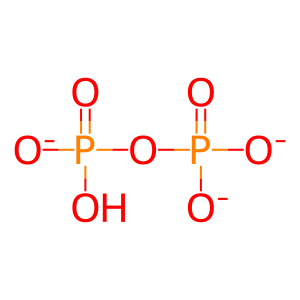
diphosphate(3-)
hydron
ADP(3-)
Reactome.org link: R-HSA-9760094