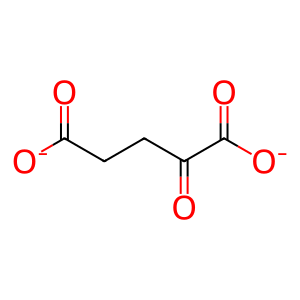
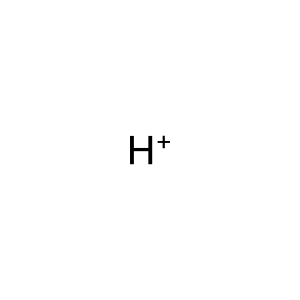
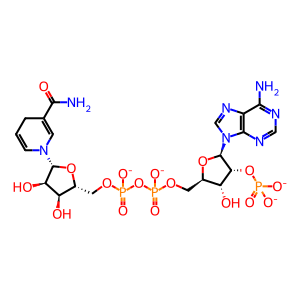
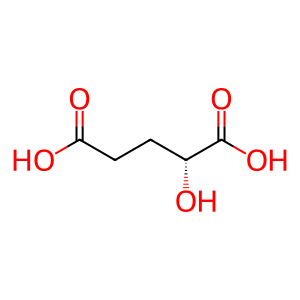
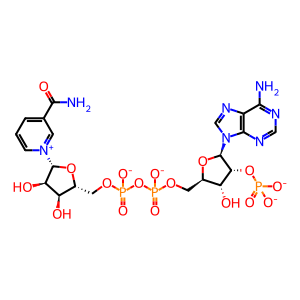
Reaction: 2-oxoglutarate + NADPH + H+ => (R)-2-hydroxyglutarate + NADP+ [mutant IDH1]
Mutant forms of IDH1 in which the arginine residue at position 132 has been replaced by histidine, cystine, leucine, or serine catalyze the reaction of 2-oxoglutarate and NADPH + H+ to form (R)-2-hydroxyglutarate and NADP+. Like normal IDH1, the mutant enzyme forms a dimer located in the cytosol (Dang et al. 2009).
Such mutations occur frequently as a somatic event in human glioblastomas (Parsons et al. 2008). Cells expressing the mutant protein accumulate elevated levels of 2-hydroxyglutarate, probably in the cytosol as IDH1 is a cytosolic enzyme. The fate of the 2-hydroxyglutarate is unclear, but the high frequency with which the mutation is found in surveys of primary tumors is consistent with the possibility that it is advantageous to the tumor cells (Dang et al. 2009).
Reaction - small molecule participants:
2HG [cytosol]
NADP+ [cytosol]
2OG [cytosol]
H+ [cytosol]
NADPH [cytosol]
Reactome.org reaction link: R-HSA-880053
======
Reaction input - small molecules:
2-oxoglutarate(2-)
hydron
NADPH(4-)
Reaction output - small molecules:
(R)-2-hydroxyglutaric acid
NADP(3-)
Reactome.org link: R-HSA-880053