Reaction: nsp14 acts as a cap N7 methyltransferase to modify SARS-CoV-2 mRNAs
- in pathway: Transcription of SARS-CoV-2 sgRNAs
SARS-CoV-2 nsp14 is a bifunctional enzyme. Apart from its exonuclease activity, which is supported by binding to nsp10, it functions independently as cap N7-methyltransferase (Saramago et al, 2021).
The genomic and subgenomic (sg) mRNAs of SARS-CoV-1 coronavirus are presumed to be capped at their 5′ end, based on studies of the mouse hepatitis virus (MHV) (Lai and Stohlman 1981) and the equine torovirus (van Vliet et al. 2002). Non-structural protein 14 (nsp14) acts as an RNA guanine-N7-methyltransferase (N7-MTase) that completes the synthesis of the cap-0 on SARS-CoV-1 mRNAs. The cap-0 represents N7-methyl guanosine connected to the 5′ nucleotide through a 5′ to 5′ triphosphate linkage, and is also known as m7G cap or m7Gppp cap. The N7-MTase domain maps to the carboxy-terminal part of nsp14 (Chen et al. 2009). Cap-0 formation requires three sequential reactions catalyzed by RNA triphosphatase (TPase), guanylyltransferase (GTase), and N7-MTase. There is no evidence that nsp14 possesses TPase and GTase activities, and no other SARS-CoV-1 proteins with these activities have been identified, so the identities of the enzymes that mediate these required steps in SARS-CoV-1 remain unknown. Based on the study of the human coronavirus 229E, non-structural protein 13 (nsp13) may have a TPase activity in addition to its established helicase activity (Ivanov and Ziebuhr 2004). In SARS-CoV-2, non-structural protein 12 (nsp12, Rdrp) has been shown to provide the GTase activity (Walker et al. 2021).
The genomic and subgenomic (sg) mRNAs of SARS-CoV-1 coronavirus are presumed to be capped at their 5′ end, based on studies of the mouse hepatitis virus (MHV) (Lai and Stohlman 1981) and the equine torovirus (van Vliet et al. 2002). Non-structural protein 14 (nsp14) acts as an RNA guanine-N7-methyltransferase (N7-MTase) that completes the synthesis of the cap-0 on SARS-CoV-1 mRNAs. The cap-0 represents N7-methyl guanosine connected to the 5′ nucleotide through a 5′ to 5′ triphosphate linkage, and is also known as m7G cap or m7Gppp cap. The N7-MTase domain maps to the carboxy-terminal part of nsp14 (Chen et al. 2009). Cap-0 formation requires three sequential reactions catalyzed by RNA triphosphatase (TPase), guanylyltransferase (GTase), and N7-MTase. There is no evidence that nsp14 possesses TPase and GTase activities, and no other SARS-CoV-1 proteins with these activities have been identified, so the identities of the enzymes that mediate these required steps in SARS-CoV-1 remain unknown. Based on the study of the human coronavirus 229E, non-structural protein 13 (nsp13) may have a TPase activity in addition to its established helicase activity (Ivanov and Ziebuhr 2004). In SARS-CoV-2, non-structural protein 12 (nsp12, Rdrp) has been shown to provide the GTase activity (Walker et al. 2021).
Reaction - small molecule participants:
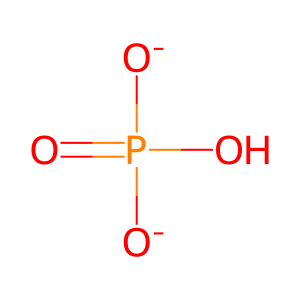
Pi [cytosol]
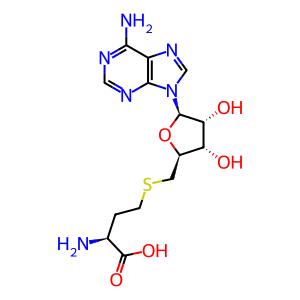
S-adenosyl-L-homocysteine [cytosol]
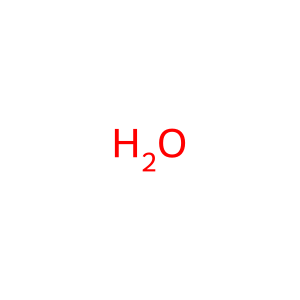
H2O [cytosol]
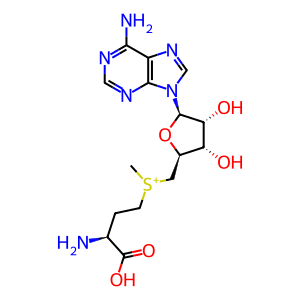
S-adenosyl-L-methionine [cytosol]
Reactome.org reaction link: R-HSA-9694476
======
Reaction input - small molecules:
water
S-adenosyl-L-methionine
Reaction output - small molecules:
hydrogenphosphate
S-adenosyl-L-homocysteine
Reactome.org link: R-HSA-9694476