Molecule: prostaglandin E2
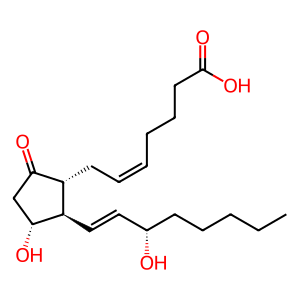
Prostaglandin F2alpha in which the hydroxy group at position 9 has been oxidised to the corresponding ketone. Prostaglandin E2 is the most common and most biologically potent of mammalian prostaglandins.
Synonyms for prostaglandin E2 :
(15S)-prostaglandin E2
(5Z,11alpha,13E,15S)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic acid
(5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate
(5Z,13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprosta-5,13-dienoate
(E,Z)-(1R,2R,3R)-7-(3-Hydroxy-2-((3S)-(3-hydroxy-1-octenyl))-5-oxocyclopentyl)-5-heptenoic acid
(Z)-7-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxyoct-1-enyl)-5-oxocyclopentyl)hept-5-enoic acid
Dinoproston
PGE2
Prostaglandin E2
U 12062
U-12,062
U-12062
Molecular Formula: C20H32O5
Molecular wt: 352.471 g/mole
Charge: 0
SMILES: CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O
InChIKey: XEYBRNLFEZDVAW-ARSRFYASSA-N
InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1
